
The goal of SynXBio Inc is to use experience in the pharmaceutical and biotech industries to generate large libraries of therapeutic biomolecules to treat emerging diseases. Our company has several decades of experience in traditional pharmaceutical development, biotech, start-up biotech, computer modeling and translating science from academic centers to production. Our science draw from several fields and currently developing a range of novel diagnostic and therapeutic discoveries.
Current projects:
- Therapeutic biomolecules to treat viral disease. SynXBio Inc has a library of over 90,000 potential biomolecules to treat diseases such as: CoVID-19, MERS, Ebola-Zaire and Zika. The initial group tested was using the viral diseases identified by the WHO as emerging disease requiring therapies.
- Therapeutic biomolecules to treat cancer.
- Therapeutic biomolecules to treat genetic disorders.
- Diagnostic biomolecules for the early detection of infectious disease.
- Diagnostic biomolecules for the early detection of genetic disease.
- Computer algorithm development to generate novel DNA, RNA and Protein based biomolecules.
| Type | Total | High Probability | |
| EBOV | Nucleic Acid | 1001189 | 40567 |
| Protein | 10000 | 263 | |
| MERS | Nucleic Acid | 752212 | 29441 |
| Protein | In Progress | NA | |
| Zika | Nucleic Acid | 250782 | 8418 |
| CoVID-19 (all variants) | Nucleic Acid | 7521370 | 49279* |
| SGI | Nucleic Acid | 136 | 58 |
*May contain replicates conserved between the variants.
Biomolecule Library
SynXBio Inc. has a large library of potential molecules for treatment and diagnosis of disease. An overview from the current projects library is shown in Table1. Once a potential disease target is identified, computer algorithms are used to determine the “total potential biomoleues” that meed very basic criteria. a sub-set of the total biomolecules are then generated using genetic algorithms. The novel molecules are then analyzed for specific traits that lead to activity allowing for high-probability molecules to be discovered. Depending on the model and the disease the computer generation steps can be accomplished in under a month. Once the high-probability molecules are identified, a subset (either random or directed) is sent out for standard synthesis. The average time to get the molecules synthesized is ranges between 1 to 3 weeks depending on scale of the project. These molecules are then tested in vitro and in vivo (Cell culture to whole organism models) to determine activity, safety and toxicology. Biomolecules that are stable, have high target specific nativity and show no signs of toxicology are then considered for full development. For a graphical overview see the “Workflow” section below.
The process does not end with the basic workflow for finding activity. The data obtained is fed back into computer models to refine the generation and selection processes. The use of high-throughput screening along with AI/ML algorithms can further refine the high probability biomolecules to a small subset for necessary in vivo studies. This can greatly decrease development cost and time to market intervals.
Work flow
The work flow for product development follows the Pharmaceutical industry standard. Start with a large pool of potential candidates, screen using inexpensive methods, retest the screening in an in vivo cell culture system and then decide to develop. If a decision to develop is made then the molecules are tested in standard models looking at safety and toxicological profile with the goal to quickly support “first-in-human” studies. This can be done quickly if following standard protocol performed by Contract Research Organizations with established history with regulatory filings. This allows for a potential to rapidly access the lucrative nucleic acid therapeutic market (See Market section below). Even more importantly, this allows for patients in need of treatments access to cutting-edge therapies.
Market Opportunities
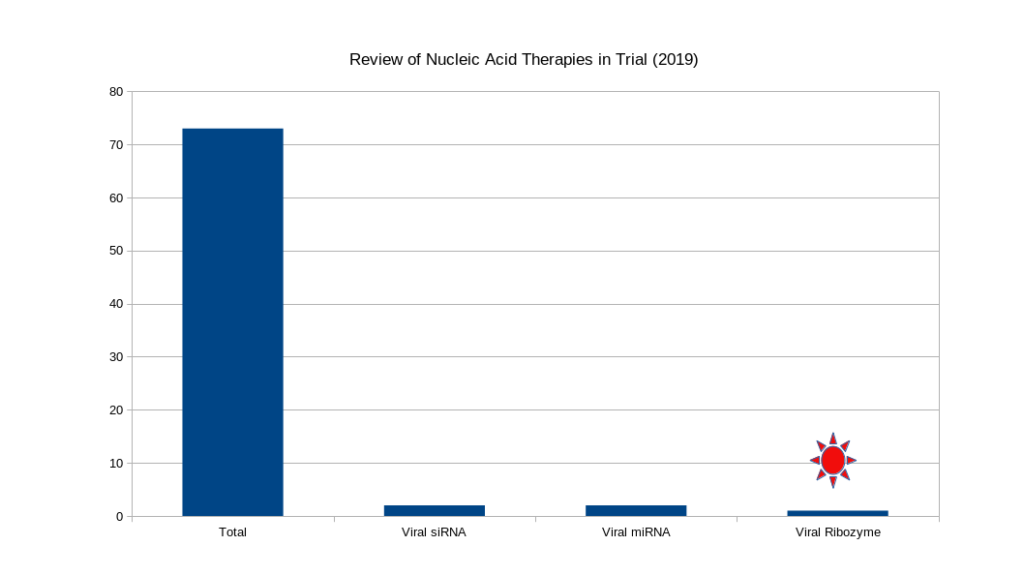
The computer generated synthetic biomolecule market is wide open. In 2019, there were around 70 nucleic acid based clinical trials register with the FDA. Of these only 5 were targeting viral infectious disease. These are treatments and not mRNA based vaccines and have a different market pressures. The nucleic acid viral therapeutic world-wide market is valued at around 40 Billion-USD and will continue to grow. SynXBio Inc. is uniquely positioned to capture a significant portion of this emerging market.